Advanced Strategies for Blood Glucose Monitoring and Diabetes Management
Advanced Strategies for Blood Glucose Monitoring and Diabetes Management
Diabetes mellitus is a multifaceted metabolic disorder characterized by chronic hyperglycemia resulting from insulin insufficiency, resistance, or a combination of both. Given its profound implications for systemic health, meticulous blood glucose monitoring is essential for effective diabetes management. It plays a critical role in optimizing treatment strategies, mitigating complications, and enhancing patient outcomes. This comprehensive guide delineates key principles and evidence-based methodologies for blood glucose testing, tailored for individuals navigating diabetes management.
1. The Imperative of Blood Glucose Monitoring
Regular glycemic assessment is foundational in diabetes care, enabling precise adjustments in pharmacotherapy, dietary intake, and lifestyle interventions. Fluctuations in blood glucose levels correlate with microvascular and macrovascular complications, necessitating proactive surveillance to maintain euglycemia. Advances in monitoring technologies, such as continuous glucose monitoring (CGM), provide dynamic glucose profiling, facilitating real-time therapeutic adjustments. Unlike traditional fingerstick methods that offer discrete glucose readings at specific moments, CGM continuously tracks glucose fluctuations throughout the day, providing a more comprehensive dataset. This enhanced granularity allows for early detection of glycemic trends, improved insulin dosing precision, and a reduction in severe hypo- or hyperglycemic episodes, ultimately improving patient adherence and long-term glycemic control.
2. Diverse Modalities of Blood Glucose Assessment
Blood glucose measurement techniques vary in their application, accuracy, and clinical utility:
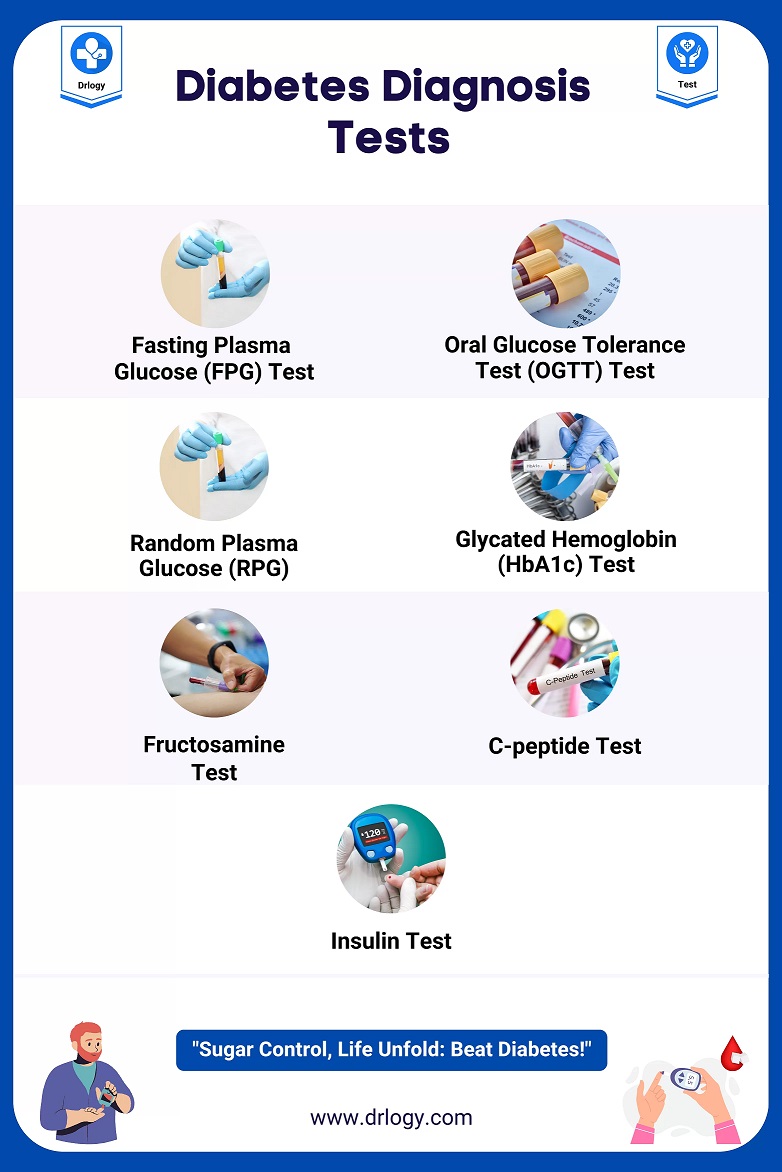
- Fasting Plasma Glucose (FPG): A benchmark test for diabetes diagnosis, requiring an overnight fast to determine basal glycemic status.
- Random Plasma Glucose (RPG): A non-fasting assessment useful for detecting severe hyperglycemia.
- Oral Glucose Tolerance Test (OGTT): Evaluates postprandial glucose handling, essential for diagnosing gestational and prediabetes states.
- Hemoglobin A1c (HbA1c): Reflects long-term glycemic control by measuring glycosylated hemoglobin, with a target typically set below 7% in most diabetic populations.
- Continuous Glucose Monitoring (CGM): Employs subcutaneous sensors to provide continuous interstitial glucose readings, particularly beneficial for individuals with Type 1 diabetes or labile glucose variability.
3. Optimizing the Accuracy of Glycemic Readings
To ensure precision in self-monitoring of blood glucose (SMBG), adherence to standardized testing protocols is paramount:
- Utilize properly calibrated glucometers and verify sensor accuracy periodically.
- Perform hand hygiene before sample collection to eliminate external contaminants.
- Employ single-use lancets and ensure test strips are stored per manufacturer specifications to prevent degradation.
- Interpret results in clinical context, considering confounding factors such as acute illness, stress, or medication effects.
4. Defining Target Glycemic Ranges
Optimal glucose targets are individualized based on patient-specific parameters, comorbidities, and treatment regimens:
- Preprandial: 80-130 mg/dL
- Postprandial (2-hour post-meal): <180 mg/dL
- HbA1c Goal: Typically <7%, though lower thresholds may be pursued in younger, healthier individuals to mitigate long-term complications.
5. Longitudinal Glucose Pattern Analysis
Systematic tracking of blood glucose trends fosters informed therapeutic decisions by providing a comprehensive view of glycemic fluctuations over time. This longitudinal data allows for more precise medication titration, enabling adjustments in insulin regimens, oral hypoglycemics, and adjunct therapies to better align with an individual’s metabolic patterns. Furthermore, trend analysis helps in identifying triggers for hyperglycemia and hypoglycemia, facilitating proactive interventions and optimizing long-term glycemic control. Emerging digital health platforms integrate SMBG and CGM data, enabling patients and clinicians to discern glycemic excursions, nocturnal hypoglycemia, and dawn phenomenon occurrences. By leveraging these insights, modifications in insulin regimens, carbohydrate intake, and exercise routines can be tailored to minimize glucose variability.
6. Pathophysiology and Clinical Implications of Glycemic Extremes
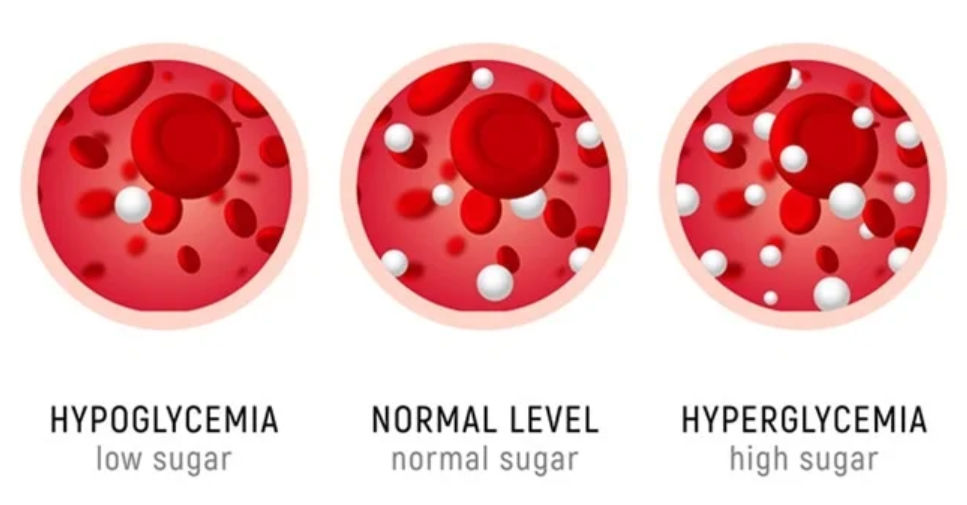
- Hyperglycemia: Prolonged elevation in glucose levels precipitates endothelial dysfunction, increasing the risk of nephropathy, neuropathy, and retinopathy. Symptoms include polydipsia, polyuria, fatigue, and blurred vision.
- Hypoglycemia: Defined as blood glucose <70 mg/dL, hypoglycemia manifests with autonomic and neuroglycopenic symptoms such as tachycardia, diaphoresis, confusion, and, in severe cases, seizure or coma. Rapid correction with 15-20 grams of glucose is recommended.
7. Dietary Strategies for Glycemic Optimization
Nutritional interventions are integral to maintaining stable glucose levels. Key dietary principles include:
- Carbohydrate Consistency: Prioritizing complex carbohydrates with a low glycemic index to prevent rapid postprandial glucose spikes.
- Protein and Fiber Integration: Enhancing meal composition with fiber-rich and protein-dense foods to modulate glucose absorption.
- Lipid Profile Considerations: Incorporating monounsaturated and polyunsaturated fats to support cardiovascular health without exacerbating insulin resistance.
8. Exercise as a Modulator of Insulin Sensitivity
Regular physical activity improves insulin responsiveness and glucose disposal, necessitating structured integration into diabetes care plans. Aerobic and resistance training synergistically enhance glycemic control, though patients on insulin or sulfonylureas should monitor for potential hypoglycemia post-exercise.
9. Pharmacologic Management and Insulin Therapy
A multitude of pharmacologic agents exist to regulate glycemia, each with distinct mechanisms of action:
- Insulin Therapy: Includes basal and bolus regimens tailored to endogenous insulin deficits.
- Oral Hypoglycemics: Such as metformin, SGLT2 inhibitors, and DPP-4 inhibitors, each targeting different metabolic pathways.
- GLP-1 Receptor Agonists: Providing both glucose-dependent insulin secretion enhancement and appetite modulation.
- Adjunctive Therapies: Emerging treatments incorporating dual-agonists and beta-cell preservation strategies hold promise for future diabetes management paradigms.
10. Interdisciplinary Coordination in Diabetes Care
Collaboration among endocrinologists, diabetes educators, dietitians, and primary care providers ensures comprehensive disease management. Endocrinologists oversee complex treatment plans, adjusting pharmacologic regimens and addressing comorbidities. Diabetes educators provide essential guidance on self-management strategies, empowering patients to monitor glucose levels and make informed decisions. Dietitians develop personalized meal plans to stabilize blood sugar and enhance nutritional outcomes. Primary care providers coordinate overall healthcare, ensuring routine screenings and preventive interventions to mitigate complications. Routine assessments, including retinal exams, renal function tests, and cardiovascular risk screenings, are imperative to detect and mitigate complications early. Patient-centered education remains paramount, empowering individuals to actively engage in self-management and lifestyle adaptation.
Conclusion
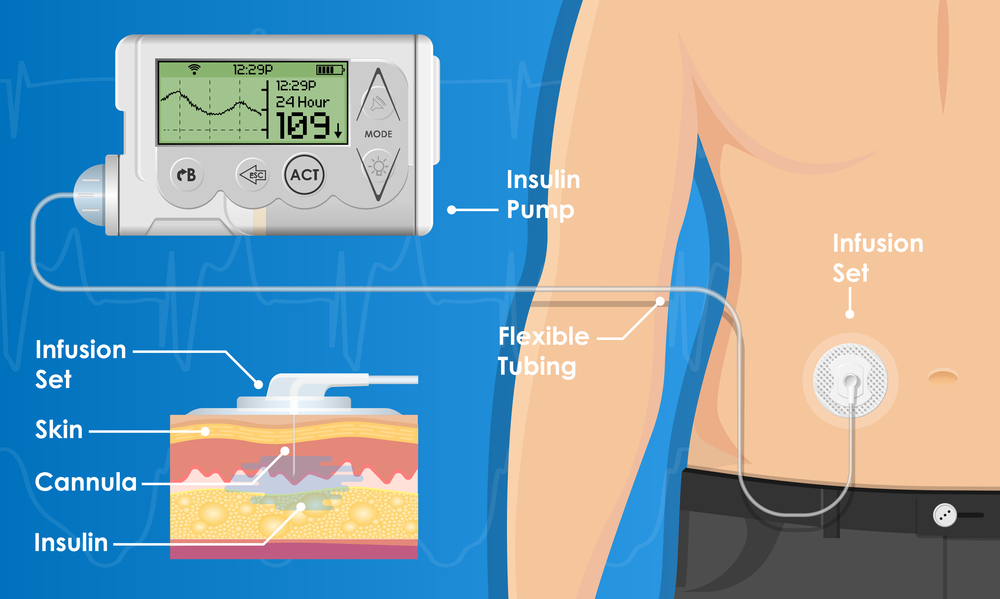
Effective diabetes management necessitates a multifaceted approach integrating precise blood glucose monitoring, evidence-based dietary strategies, individualized pharmacotherapy, and interdisciplinary collaboration. Advances in glucose-sensing technologies and therapeutic innovations continue to refine clinical practices, underscoring the importance of continual adaptation in diabetes care. For instance, the advent of hybrid closed-loop insulin delivery systems, as demonstrated in recent clinical trials, has significantly improved glycemic control in individuals with Type 1 diabetes by automating insulin dosing based on real-time CGM data. Additionally, research on beta-cell regeneration therapies and personalized medicine approaches, such as gene editing and immune modulation, holds promise for future diabetes management paradigms. By adhering to these advanced principles, individuals with diabetes can optimize glycemic control, reduce complication risks, and enhance their overall quality of life.